Background:
Mutations in thenucleophosmin1 (NPM1) are considered as founder mutations in acute myeloid leukemia (AML). NPM1 mutations in exon 12 occur in about 60% adult AML patients with normal karyotype. Among all NPM1 subtypes, type A (69.15%), type B (7.41%) and type D (7.41%) are found to be most frequent. The evaluation of minimal residual disease (MRD) has been considered critical to more precisely define AML response to clinical treatment, thereby refining disease risk stratification. Currently, AML with NPM1 gene mutations is recognized as a distinct entity, due to its unique clinical features. A large body of evidence demonstrated a significant independent prognosis factor of molecular MRD monitoring in NPM1-mutated AML.
Objectives:
Here we present GeneXpert NPM1 prototype, a fast and promising test for use in AML mutation detection (this prototype is for research use only, not for use in diagnostic procedures, and not reviewed by any regulatory body). NPM1 prototype is an easy-to-use-sample in/results out, and highly sensitive assay. The turnaround time is as fast as less than 3 hours. NPM1 prototype is designed for the quantitative detection of NPM1 mutant mRNA transcripts (types A, B and D in exon 12) and the ABL endogenous control mRNA transcript in peripheral blood of AML patients. This test is also designed for use as an aid in the monitoring of mutant NPM1 mRNA transcript levels in patients diagnosed with NPM1-mutated AML. This test does not differentiate between A, B or D type mutant NPM1 transcripts and does not detect or monitor other rare types of mutant NPM1.
Results:
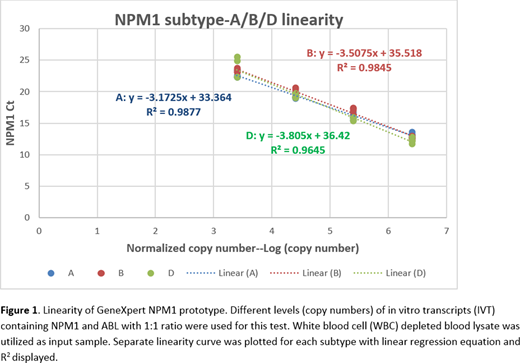
Through assay optimization, we were able to reach limit of detection (LoD) within 0.05% for three NPM1 subtypes among all screened EDTA donor blood samples. Ct-copy number regression curve aligns well among three subtypes, within detection range. Our assay specificity has been above 95% among 30 screened EDTA donor blood samples.
Conclusion:
In summary, GeneXpert NPM1 prototype shows promise as a fast test for use in AML mutation detection.The presented NPM1 prototype has a great advantage covering most prevalent NPM1 mutation types up to 94% of all NPM1 subtypes. Our results demonstrated that our prototype shows satisfactory assay specificity and sensitivity. Clinical samples will be included for our clinical validation in the future.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal